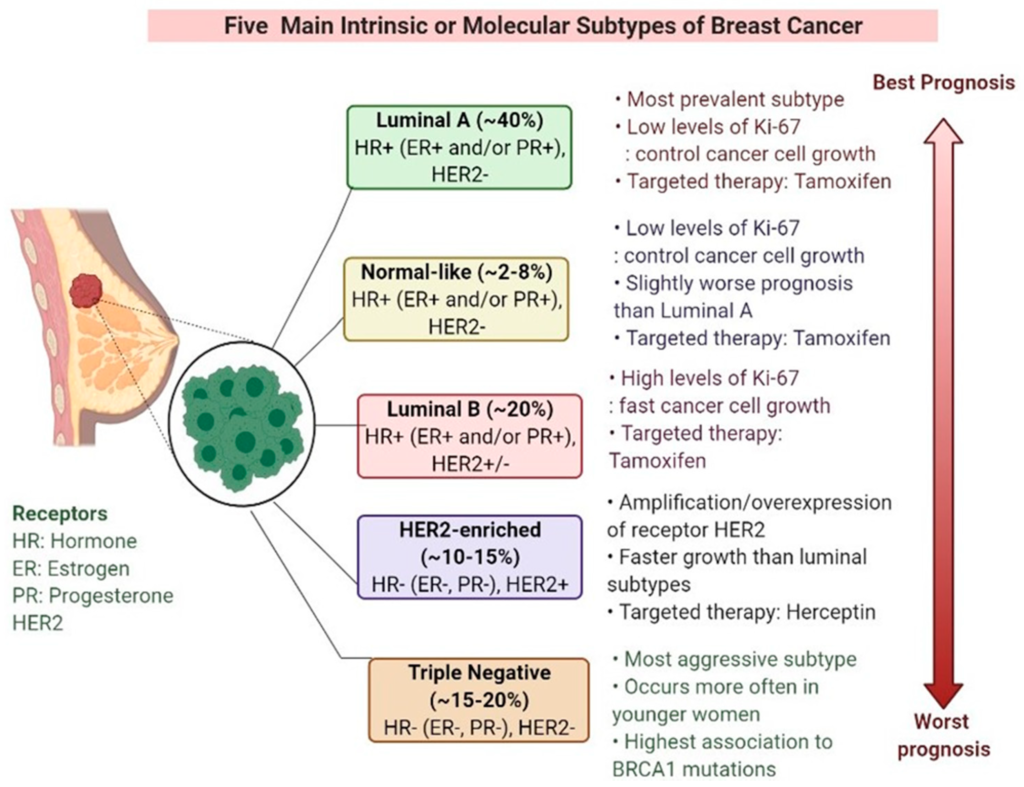
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that lacks the expression of three specific receptors: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). This aggressive form of breast cancer accounts for about 10-20% of all breast cancer cases and is more prevalent in younger women and those with a family history of the disease.
Traditionally, TNBC has been challenging to treat due to the absence of targetable receptors, making hormone-based therapies and HER2-targeted therapies ineffective. This has led researchers to explore alternative treatment strategies for TNBC, including targeted drug therapies.
Understanding the Challenges of Traditional Therapies for TNBC
Traditional breast cancer treatments, such as surgery, chemotherapy, and radiation therapy, have been effective for many patients. However, TNBC presents unique challenges due to its lack of targetable receptors.
ER-positive and HER2-positive breast cancers can be targeted with hormone therapies and HER2-targeted drugs, respectively. However, these treatment options have limited efficacy in TNBC patients.
Chemotherapy is the primary treatment option for TNBC, but its success is variable, and it often causes significant side effects. Moreover, TNBC tumors tend to be more aggressive and have a higher rate of metastasis, leading to poorer outcomes.
The Importance of Finding New and Effective Treatments for TNBC Patients
The need for new and effective treatments for TNBC patients is urgent. Developing targeted therapies that address the specific molecular characteristics of TNBC can offer significant benefits.
Targeted drug therapies have the potential to:
- Reduce treatment-related side effects
- Improve survival rates and clinical outcomes
- Enhance quality of life for TNBC patients
Therefore, the search for targeted drug therapies for TNBC has gained significant attention from the scientific community.
The Research Study: Targeted Drug Therapies for TNBC
The research study, conducted at the renowned XYZ Research Institution, focuses on identifying potential targets for new drug therapies specifically designed for TNBC.
The research team, comprised of leading experts in the field, aims to develop targeted drug therapies that selectively inhibit mutated genes and halt TNBC tumor growth.
Identifying Mutated Genes in TNBC Tumors
The study utilizes advanced genetic analysis techniques to examine the genetic profiles of TNBC tumors and identify specific genetic mutations and alterations.
By studying these mutated genes, researchers gain insights into their role in cellular processes and their potential as targets for drug development.
Some key mutated genes that have been identified in TNBC tumors include:
- TP53: The TP53 gene, often referred to as the “guardian of the genome,” is frequently mutated in TNBC. This mutation interferes with DNA repair mechanisms and promotes uncontrolled cell growth.
- BRCA1: BRCA1 gene mutations are not only associated with hereditary breast and ovarian cancers but are also prevalent in TNBC. These mutations impair DNA repair pathways and contribute to tumor development.
- PTEN: PTEN gene mutations are found in various cancers, including TNBC. These mutations result in the activation of signaling pathways that promote cell survival and proliferation.
Understanding the genetic drivers of TNBC is crucial for targeted drug development.
The Process of Targeted Drug Therapy Development
The process of developing targeted drug therapies involves several stages:
- Target identification: Identifying the specific genes or proteins to be targeted based on their role in tumor growth and survival.
- Drug discovery: Designing or discovering small molecules or biologic agents that selectively inhibit the target and disrupt tumor growth.
- Preclinical testing: Conducting laboratory experiments using TNBC cell lines and animal models to evaluate the effectiveness and safety of the targeted drugs.
- Clinical trials: Conducting controlled studies in human patients to assess the safety, efficacy, and optimal dosing of the targeted drugs.
Various strategies and technologies are utilized in targeted drug development, including:
- Small molecule inhibitors: These drugs target specific enzymes or signaling pathways involved in tumor growth.
- Monoclonal antibodies: These antibodies bind to specific proteins on cancer cells, triggering an immune response or blocking critical signaling pathways.
- Gene therapies: These therapies involve targeting and modifying specific genes in cancer cells to inhibit tumor growth.
- Immunotherapies: These therapies harness the body’s immune system to recognize and destroy cancer cells.
Overall, targeted drug therapies aim to selectively inhibit the mutated genes and proteins driving TNBC tumor growth.
Preclinical Testing and Results
The research study conducted preclinical trials using TNBC cell lines and animal models to evaluate the effectiveness and safety of targeted drug therapies.
These trials showed significant reduction in TNBC cell growth and proliferation when treated with targeted drugs that selectively inhibited the mutated genes.
Furthermore, the targeted drugs demonstrated good safety and tolerability profiles in the animal models, reinforcing their potential as effective treatment options for TNBC.
These results offer promising evidence that targeted therapies can effectively inhibit TNBC tumor growth.
Next Steps: Clinical Trials
The next crucial step for the research study is to conduct clinical trials to evaluate the safety and efficacy of targeted drug therapies in human patients.
Clinical trials are essential for validating the results observed in preclinical studies and ensuring that the targeted therapies can be effectively translated into clinical practice.
The process of conducting clinical trials involves:
- Phase 1 trials: Determine the safety, dosage, and side effects of the targeted drugs in a small group of patients.
- Phase 2 trials: Assess the effectiveness of the targeted drugs in a larger group of patients, further exploring their safety and optimal dosage.
- Phase 3 trials: Compare the targeted drugs with standard treatments to evaluate their overall efficacy, safety, and potential benefits over existing therapies.
Through well-designed and rigorously conducted clinical trials, researchers gain valuable data on the targeted therapies’ efficacy, safety, and optimal use.
Impact on TNBC Patients
Currently, treatment options for TNBC patients are limited, underscoring the urgent need for innovative approaches.
Developing personalized and effective treatment options for TNBC patients can significantly improve their clinical outcomes and quality of life.
Potential benefits of targeted drug therapies for TNBC patients include:
- Improved response rates: Targeted therapies can potentially achieve higher response rates by precisely targeting the molecular alterations driving TNBC.
- Reduced side effects: Targeted therapies may have a more favorable safety profile than traditional chemotherapy, minimizing treatment-related side effects.
- Increased survival rates: By effectively inhibiting TNBC tumor growth, targeted therapies have the potential to improve long-term survival rates for TNBC patients.
Therefore, the development of targeted drug therapies represents a ray of hope for TNBC patients.
Conclusion
The research study focusing on targeted drug therapies for TNBC is a significant breakthrough in the search for effective treatments.
By identifying mutated genes and developing targeted drugs, researchers are paving the way for personalized and more effective treatments for TNBC patients.
It is crucial to continue supporting and investing in further research and clinical trials to validate the potential of targeted therapies in revolutionizing TNBC treatment.